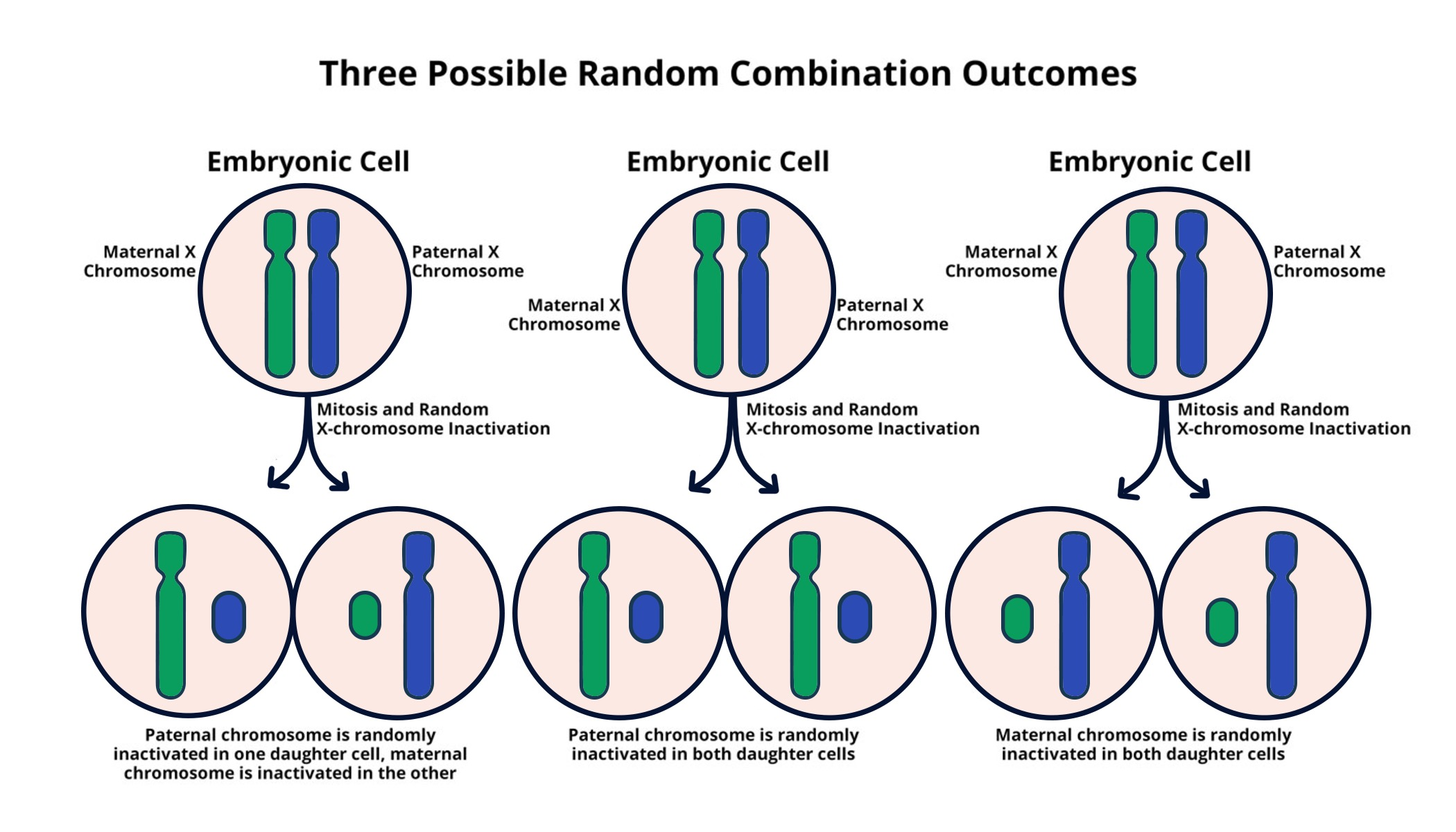
X chromosome inactivation is a fascinating genetic process that plays a crucial role in how female cells manage their two X chromosomes. Unlike males, females possess two copies of the X chromosome, leading to the necessity of inactivating one to prevent an overload of X-linked gene expression. This chromosomal silencing, a complex mechanism investigated by researchers such as Jeannie T. Lee, is pivotal in understanding various genetic disorders, including Fragile X syndrome and Rett syndrome. Recent studies reveal how a gelatinous substance influences this inactivation, potentially paving the way for innovative treatments. By unraveling the mysteries of X chromosome inactivation, scientists hope to unlock new pathways for therapeutic interventions targeting these challenging genetic conditions.
The silencing of one X chromosome in females, known as X chromosome inactivation, serves as a vital biological function that ensures genetic balance. This process, which involves chromosomal coating and the action of specific molecules, allows cells to become less reliant on the potentially deleterious genes present on the inactivated X chromosome. Among the groundbreaking work being conducted to elucidate these mechanisms is research led by Jeannie T. Lee at Harvard Medical School, where insights into chromosomal behavior could lead to therapies for neurodevelopmental disorders. This genetic phenomenon holds significant implications not only for females affected by conditions like Fragile X syndrome and Rett syndrome but also for the broader gene dynamics in males, illustrating a universal aspect of gene regulation across sexes.
Understanding X Chromosome Inactivation: A Cellular Mechanism
X chromosome inactivation is a crucial process that occurs in female mammals to balance gene dosage between sexes. Since females possess two copies of the X chromosome, while males have just one, evolutionary mechanisms have developed to ensure that only one X chromosome in each cell is activated, thus maintaining a functional equilibrium. This intricate form of chromosomal silencing is essential for proper cellular function and development. This phenomenon, discovered in the early half of the 20th century, showcases how genetic regulation can adapt to the differences presented by sex-linked chromosomes, ensuring that females do not express double the amount of genes typically encoded on this important chromosome.
The process of X chromosome inactivation hinges on a long non-coding RNA known as Xist. This RNA plays a pivotal role, initiating the inactivation process and altering the structure of chromatin surrounding the X chromosome. As research led by scientists like Jeannie T. Lee demonstrates, the gelatinous-like substance surrounding chromosomes—likened to Jell-O—serves as a medium through which Xist interacts, facilitating the spatial rearrangements necessary for successful silencing. The implications of this discovery extend beyond basic biology, heralding new approaches for treating genetic disorders linked to the X chromosome.
Frequently Asked Questions
What is X chromosome inactivation and how does it relate to genetic disorders?
X chromosome inactivation (XCI) is a biological process in females where one of the two X chromosomes is randomly silenced to equalize gene dosage between males and females. This process is crucial for preventing genetic disorders linked to mutations on the X chromosome, including Fragile X syndrome and Rett syndrome, as it ensures that excessive gene expression does not occur from the presence of two Xs.
How does Jeannie T. Lee’s research contribute to understanding X chromosome inactivation?
Jeannie T. Lee’s research is pivotal in deciphering the mechanisms of X chromosome inactivation. Her studies demonstrate how a specialized RNA molecule called Xist helps orchestrate chromosomal silencing, revealing potential therapeutic pathways for conditions like Fragile X syndrome and Rett syndrome by activating the silenced genes on the X chromosome.
What role does the ‘Jell-O-like substance’ play in X chromosome inactivation?
The ‘Jell-O-like substance’ refers to a gelatinous material that coats chromosomes and facilitates X chromosome inactivation. According to research by Jeannie T. Lee, this substance helps create a flexible environment around the X chromosome, allowing Xist and other molecules to infiltrate and silence the chromosome effectively, thereby impacting gene expression related to genetic disorders.
Can X chromosome inactivation techniques be used to treat Fragile X syndrome?
Yes, techniques to modify X chromosome inactivation are being explored as potential treatments for Fragile X syndrome. By unsilencing the mutated gene on the inactive X chromosome, researchers, including Jeannie T. Lee’s team, aim to restore normal function to cells affected by this genetic disorder.
What is the potential impact of unsilencing inactivated X chromosomes on genetic disorders?
Unsilencing inactivated X chromosomes could revolutionize the treatment of genetic disorders like Fragile X syndrome and Rett syndrome. By activating the healthy gene on the inactive chromosome, it could potentially cure these disorders with minimal side effects, as suggested by Jeannie T. Lee’s ongoing research.
Are males affected by processes related to X chromosome inactivation?
While males have only one X chromosome and do not undergo X chromosome inactivation, certain mutations can still lead to gene silencing. Conditions like Fragile X syndrome illustrate how X-linked mutations can influence both sexes, highlighting the importance of understanding XCI processes in therapeutic approaches.
What are some implications of Jeannie T. Lee’s findings on chromosomal silencing for future research?
Jeannie T. Lee’s findings on chromosomal silencing provide a foundation for developing targeted therapies for genetic disorders associated with the X chromosome. As research progresses, understanding the nuances of X chromosome inactivation can lead to innovative treatments that could address not only Fragile X syndrome and Rett syndrome but potentially other X-linked conditions as well.
| Key Point | Details |
|---|---|
| X Chromosome Challenge | Females have two X chromosomes while males have one, leading to the need for X chromosome inactivation. |
| Role of Xist | A gene on the X chromosome produces an RNA molecule called Xist, which is essential for inactivation. |
| Mechanism of Inactivation | Xist changes the material properties of the chromatin, allowing access to other molecules that contribute to inactivation. |
| Potential Therapies | Research aims to develop treatments for Fragile X and Rett syndromes by unsilencing inactivated X-linked genes. |
| Clinical Implications | Work may enable the release of healthy gene functions from inactivated X chromosomes with minimal side effects. |
| Funding and Support | The research has been supported by the National Institutes of Health for 25 years. |
Summary
X chromosome inactivation is a crucial biological process that allows females to manage the presence of two X chromosomes effectively. This fascinating mechanism, explored through Jeannie T. Lee’s groundbreaking research, unveils how Xist RNA mediates the silencing of one X chromosome. The findings indicate that significant progress in understanding this process may lead to innovative treatments for genetic disorders such as Fragile X and Rett syndromes, representing a significant step toward novel therapies that could potentially alleviate the burdens of these conditions. As researchers refine their approaches and move toward clinical trials, the promise of targeting X chromosome inactivation continues to inspire hope in genetic medicine.